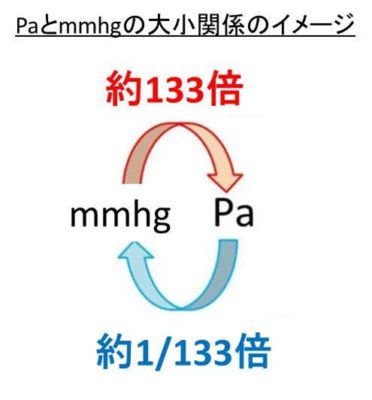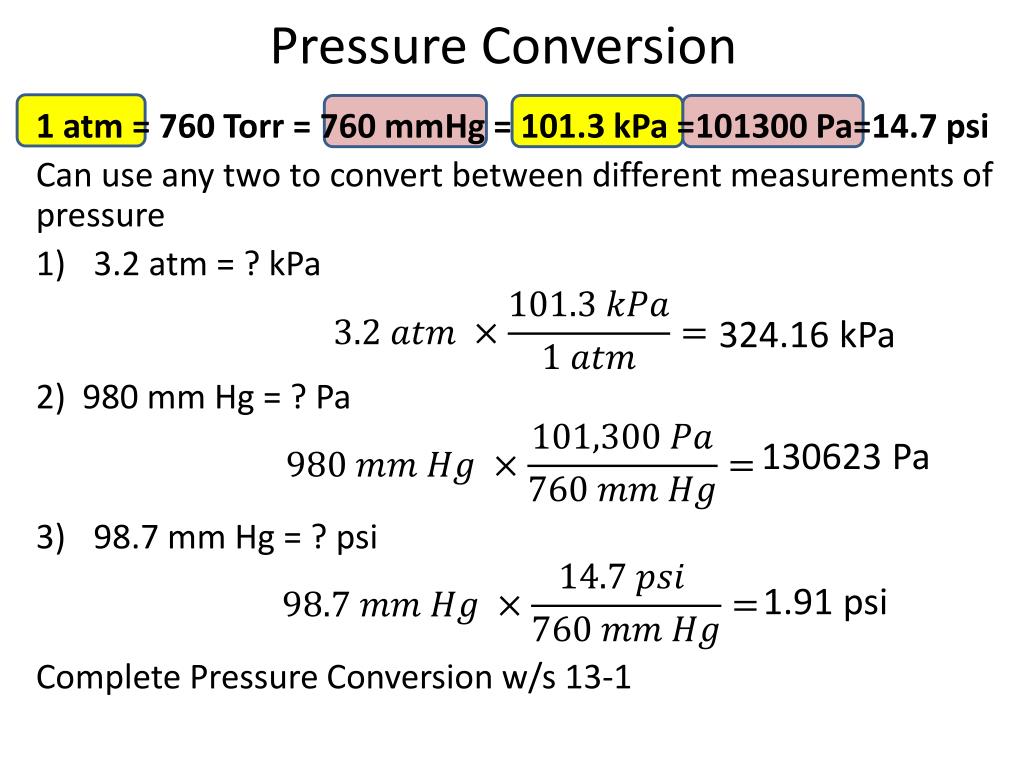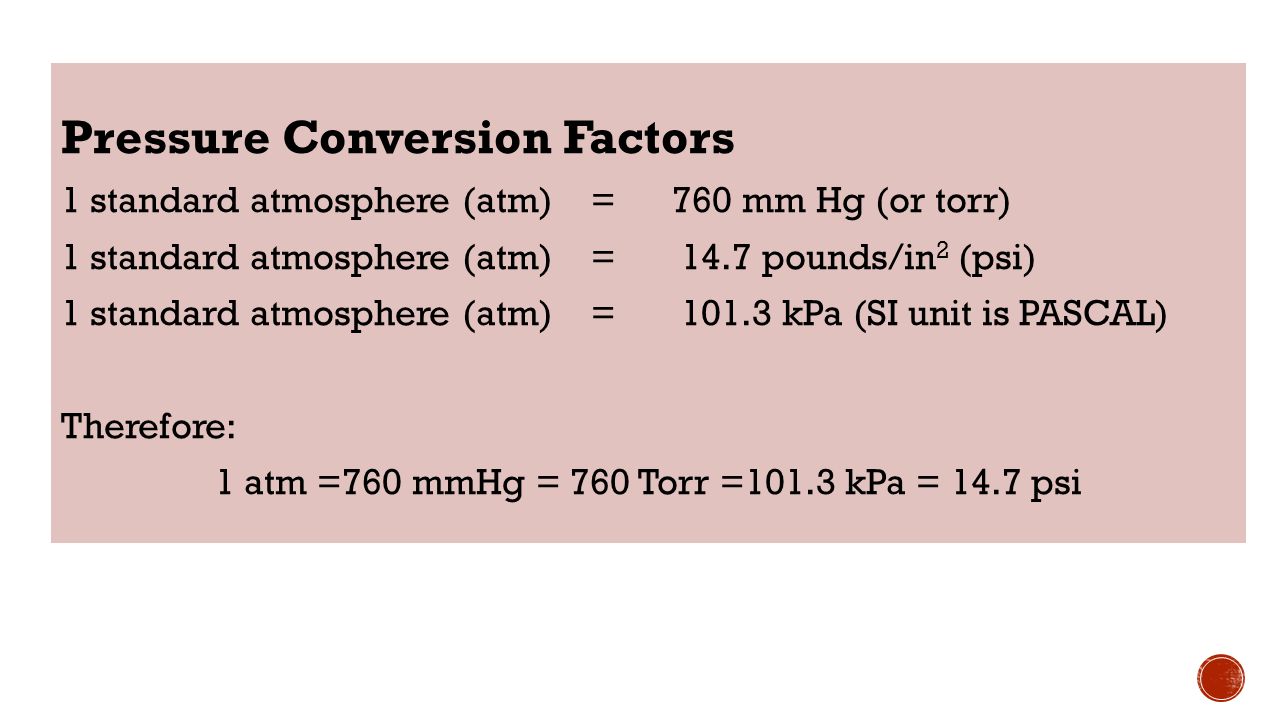
Topic#33: Gas Laws EQ: How do we calculate the relations between the pressure, temperature, and volume of gases? - ppt video online download

Gases Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. - ppt download

24-h ICP (mmHg) and PA (mmHg) in IIH patients with PT (n = 5) and NPT... | Download Scientific Diagram

pressure, conversion units into defferent units,atm,bar,torr,psi,Pascal,mmHg, numerical,and examples - YouTube

Chapter 10 The Kinetic-Molecular Theory is based on the idea that particles of matter are always in motion. The constant motion of particles mean they. - ppt download
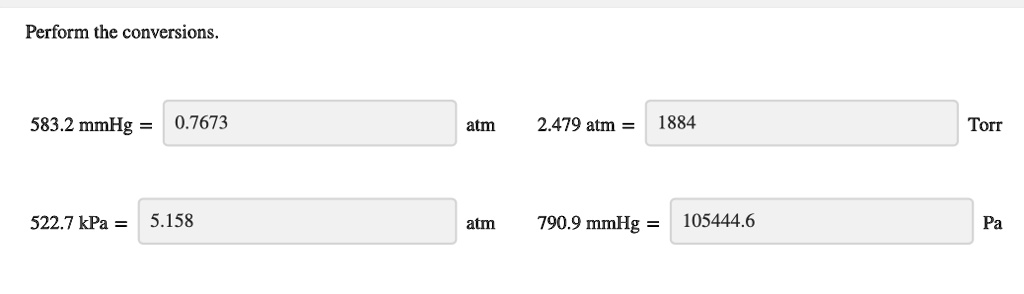
SOLVED: Perform the conversions 583.2 mmHg 0.7673 atm 2.479 atm 1884 Torr 522, kPa 5.158 atm 790.9 mmHg 105444.6 Pa
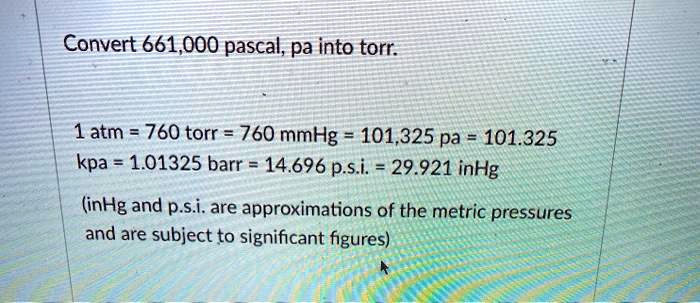
SOLVED: Convert 661,000 pascal, pa into torr 1 atm 760 torr = 760 mmHg 101.,325 pa 101.325 kpa 1.01325 barr 14.696 p.si 29.921 inHg (inHg and p.s.i. are approximations of the metric pressures and are subject to significant figures)

SOLVED:Make the indicated pressure conversions. a. 1.54 ×10^5 Pa to atmospheres b. 1.21 atm to pascals c. 97,345 Pa to mm Hg d. 1.32 kPa to pascals
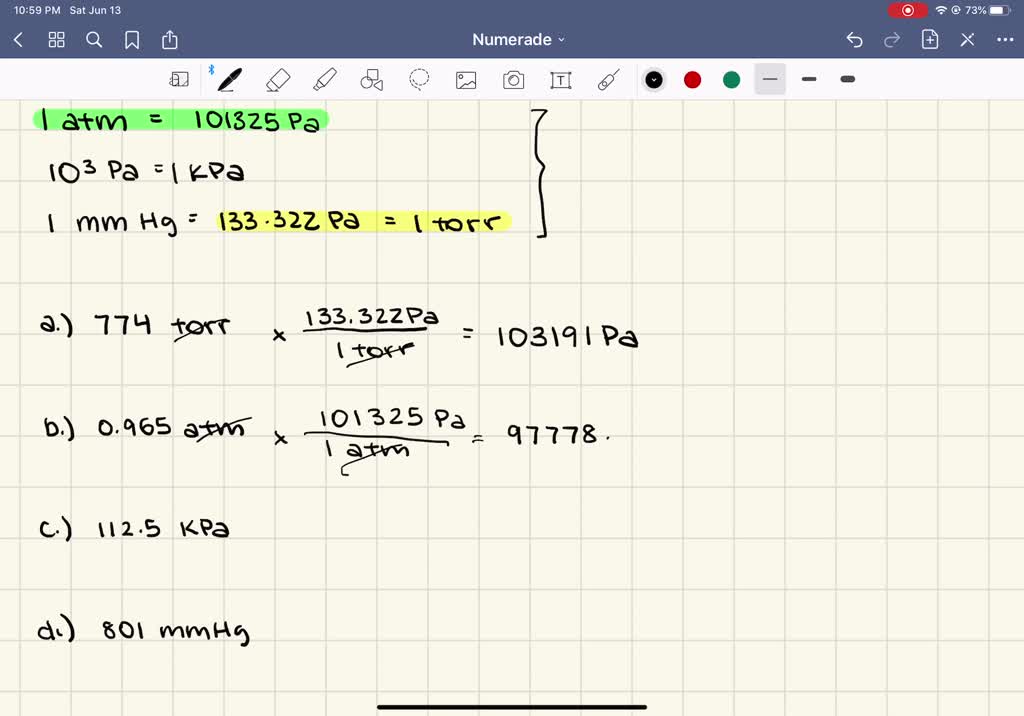
SOLVED:Convert the following pressures into pascals. a. 774 torr b. 0.965 atm c. 112.5 kPa d. 801 mm Hg

homework and exercises - How is formula for converting pressure from mmHg to Pa derived? - Physics Stack Exchange